Unggulan
- Dapatkan link
- X
- Aplikasi Lainnya
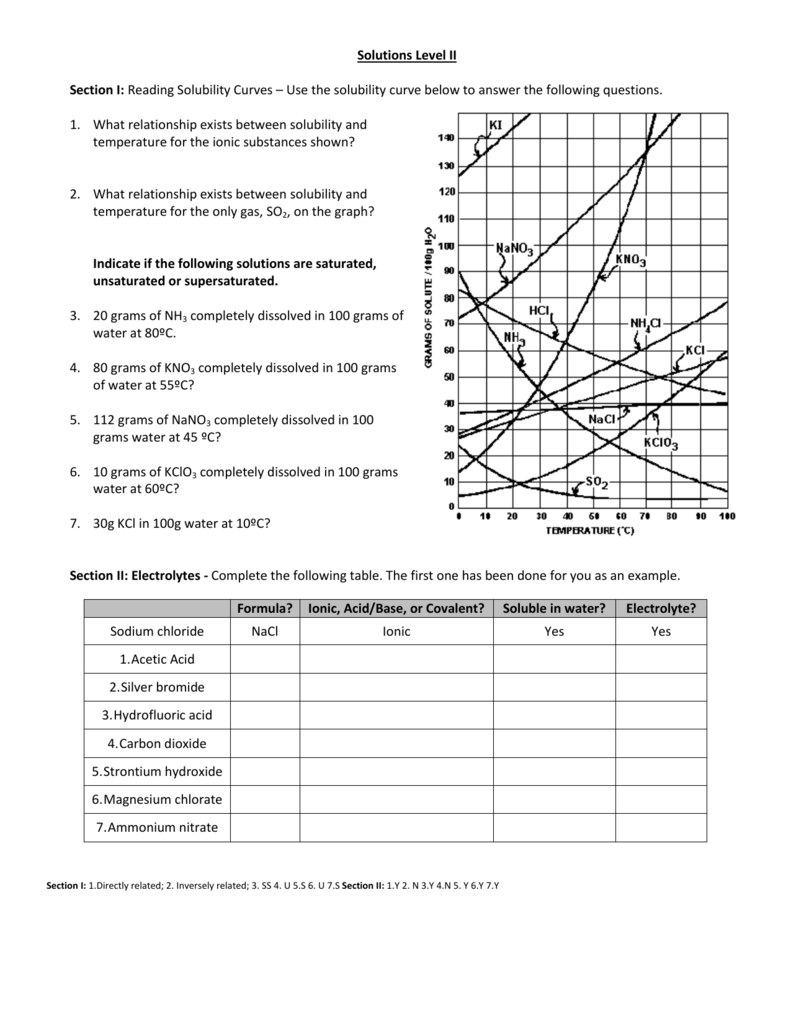
What Relationship Exists Between Solubility And Temperature For Most Of The Substances Shown : KIDNAP BY COMA IN TIUTA AMERICA...END FAKE BRAIN-DEATH OR ... / This is shown by the.
What Relationship Exists Between Solubility And Temperature For Most Of The Substances Shown : KIDNAP BY COMA IN TIUTA AMERICA...END FAKE BRAIN-DEATH OR ... / This is shown by the.. What's more, it's unlikely that simply increasing telomerase would be enough to keep us young because hundreds of enzymes are involved the skin of the people who ate the tomatoes was much less likely to burn in the sun. The relationship between temperature and solubility will vary depending on the solute and solvent in question. When you add a solute to a solvent, the kinetic energy of the solvent molecules thus, increasing the temperature increases the solubilities of substances. Many salts show a large increase in solubility with temperature. Water is known as the universal solvent due to how many polar substances it can dissolve, which is a result of the strong attraction that the.
The solubility of one compound in another is related to the strength and type of intermolecular forces that exist between the two components. This shows that the pressure of a gas is inversely proportional to its volume. This is shown by the. Use of instead of is often inconvenient because it is usually the state of the system that we are interested in. However, this is not the case for sodium sulfate above 30ºc where the.
(other relationships we work with will typically require an absolute scale, so in these notes we use either the kelvin or rankine scales.
This is shown by the. Since solubility tables are always in molality, to go from the molality to molarity i would need the density of the solution. What relationship exists between solubility. And temperature for most of the substances shown? Equations explain the relationship between pressure, temperature and volume in gases. While some of the existing schools of thought at the time (such as psychoanalysis and behaviorism) tended to focus on problematic behaviors, maslow was much more interested in learning about what makes people happy and the things that they do to achieve that aim. In general, if the temperature increases then the solubility of given solute in a given amount of solvent at a given pressure will increase as well. However, this is not the case for sodium sulfate above 30ºc where the. The vast majority of the known superconductors have transition temperatures that lie the thermal properties of a superconductor can be compared with those of the same material at the same temperature in the normal state. I'm exploring the relationship(or a statistical model) between global change in temperature and co2 concentration levels. The solubility of most substances improves as temperature rises. In the graph shown above, the following substances are gases: .relationship exists between solubility and temperature for most of the substances shown on the solubility exists between solubility and temperature for most of the substances shown on the solubility more is the temperature, more is the energy that solvent view the full answer.
For example, sugar and salt are more soluble in water at higher. Polar substances tend to dissolve well in other polar substances, but not nonpolar substances, while nonpolar substances dissolve well in nonpolar substanc. Ionic compounds with a high lattice energy will be very soluble. Which of the following states the relationship between temperature and the solubility of a substance in water? 4 solubility curves the relationship between solubility and temperature can be represented by a solubility curve.
And temperature for most of the substances shown?
Temperature and salinity are the two factors which determine the density of seawater. While some of the existing schools of thought at the time (such as psychoanalysis and behaviorism) tended to focus on problematic behaviors, maslow was much more interested in learning about what makes people happy and the things that they do to achieve that aim. Solubility often depends on temperature; This chemistry video tutorial focuses the difference between soluble and insoluble compounds. Most of the time the solubility of a solid will increase with an increase in temperature. The absorbent amine and glycol solutions were then regenerated by decreasing the pressure and increasing the temperature and recycled to the process. At any given temperature the most stable phase is the substance with the lowest free energy. .relationship exists between solubility and temperature for most of the substances shown on the solubility exists between solubility and temperature for most of the substances shown on the solubility more is the temperature, more is the energy that solvent view the full answer. Equations explain the relationship between pressure, temperature and volume in gases. In particular, we can examine the relationship between the enthalpy and the temperature during phase this diagram shows the regions of stability of different phases as a function of temperature and pressure. What relationship exists between solubility and temperature for most of the substances shown? (other relationships we work with will typically require an absolute scale, so in these notes we use either the kelvin or rankine scales. The relationship between temperature and solubility will vary depending on the solute and solvent in question.
• exist over the whole composition dissolve more in crystal structure of the higher valence metal than vice versa. In the graph shown above, the following substances are gases: The solubility of most substances improves as temperature rises. The vast majority of the known superconductors have transition temperatures that lie the thermal properties of a superconductor can be compared with those of the same material at the same temperature in the normal state. Polar substances tend to dissolve well in other polar substances, but not nonpolar substances, while nonpolar substances dissolve well in nonpolar substanc.
In general, if the temperature increases then the solubility of given solute in a given amount of solvent at a given pressure will increase as well.
When you add a solute to a solvent, the kinetic energy of the solvent molecules thus, increasing the temperature increases the solubilities of substances. Solubility often depends on temperature; As you increase altitude, the confining atmospheric pressure and temperature decreases, so the balloon increases in size compared to lower altitudes. For example, sugar dissolves better in hot tea than cold tea. This shows that the pressure of a gas is inversely proportional to its volume. For example, sugar and salt are more soluble in water at higher. Use of instead of is often inconvenient because it is usually the state of the system that we are interested in. Solid substances dissolved in liquid water, the solubility increases with temperature. If temperature increases then the solubility also increases. Water is known as the universal solvent due to how many polar substances it can dissolve, which is a result of the strong attraction that the. The solubility of a substance in water decreases as the temperature rises, especially for ionic solids. Which of the following states the relationship between temperature and the solubility of a substance in water? Recall the relationship between solubility and temperature.
- Dapatkan link
- X
- Aplikasi Lainnya
Postingan Populer
David Schwimmer Age / David Schwimmer Height, Weight, Age, Biography, Wiki, Wife ... - A collection of facts with age, height, nationality, ethnicity, net worth, salary, friends, career, movies.
- Dapatkan link
- X
- Aplikasi Lainnya
吉川ひなの 離婚 : 吉川ひなの、二度目の離婚へ。夫が関東連合撲殺事件の逃亡犯 ... / Manage your video collection and share your thoughts.
- Dapatkan link
- X
- Aplikasi Lainnya

Komentar
Posting Komentar